| View previous topic :: View next topic |
| Author |
Message |
mcstargazer
Joined: 07 Nov 2006
Posts: 4
|
 Posted: Tue Nov 07, 2006 11:24 pm Post subject: An Improved "Block of Wood" Posted: Tue Nov 07, 2006 11:24 pm Post subject: An Improved "Block of Wood" |
 |
|
Hello,
I keep telling myself that I am going to follow up on an experiment I did last summer with enhancements. I also keep intending to create a website to post the results of these experiments. However as a graduate student I can never seem to come up with the time. So here are the initial experimental results in text. Does anybody with more time want to try the enhancements?
***** Background *****
I have been using a block of wood as an insulator in my CooKit. While other arrangements may be made using sticks or stones, the block is very appealing to me because of its stability in holding the pot, and the fact that it is only one item to keep track of. Can it be improved? My idea is to create a block with greater resistance to heat flow. The design I came up with uses layers of corrugated cardboard. The dead-air space in the cardboard should slow the flow of heat through the block, thus allowing an actively cooking pot to reach a higher temperature. Also, if clouds temporarily block the sun, the pot should stay hot longer.
***** Construction Details *****
1) Several layers of corrugated cardboard are cut to size to make a block as nearly the same size as the control block of wood as is possible.
2) Each cardboard layer has the open air spaces on opposite ends sealed with cellophane tape. This is to prevent the flow of air out of the blocks.
***** Experimental Design *****
In order to make this experiment as sound as possible, I tried to re-create the exact same conditions each time I re-ran it. The following steps were followed:
1) Each time, I heated 1 liter of water to boiling over a conventional stove in a 3 liter black enameled roaster pan.
2) I then quickly placed a digital cooking thermometer probe into the water, put a lid on the pot, bagged the pot, and then placed it over either a block of wood or cardboard. The block was placed on an indoor tile floor and I tried to keep the air temperature as steady as possible.
3) I recorded the time it took for the water in the pot to cool through 18 degrees Celsius.
***** Experimental Results *****
Test #1: Block of Wood
Ambient Air Temp - 24C
Beginning Water Temp - 95C
Ending Water Temp - 77C
Change in Water Temp - 18C
Time to cool 18C - 14.00 Minutes
Test #2: Cardboard Block
Ambient Air Temp - 23C
Beginning Water Temp - 93C
Ending Water Temp - 75C
Change in Water Temp - 18C
Time to cool 18C - 19.00 Minutes
Test #3: Cardboard Block
Ambient Air Temp - 22C
Beginning Water Temp - 92C
Ending Water Temp - 74C
Change in Water Temp - 18C
Time to cool 18C - 18.13 Minutes
Test #4: Block of Wood
Ambient Air Temp - 21C
Beginning Water Temp - 93C
Ending Water Temp - 75C
Change in Water Temp - 18C
Time to cool 18C - 14.50 Minutes
Test #5: Cardboard Block
Ambient Air Temp - 22C
Beginning Water Temp - 93C
Ending Water Temp - 75C
Change in Water Temp - 18C
Time to cool 18C - 19.02 Minutes
Test #6: Block of Wood
Ambient Air Temp - 23C
Beginning Water Temp - 93C
Ending Water Temp - 75C
Change in Water Temp - 18C
Time to cool 18C - 15.47 Minutes
***** Discussion *****
The general trend seems to indicate that the cardboard block is more efficient at keeping heat in the pot than the wooden block. While this is not a statistically significant number of tests, two comparisons may be made in the data above to supporting this claim.
1) Comparison of single tests #2 and #6 seem to indicate an 18% increase in time that the pot was held above 75C with the cardboard. These are at the same ambient temperature and beginning and ending temperatures for both the block of wood and the cardboard block.
2) Comparing tests #1 and #2 is also fruitful. Test one with the wooden block should have taken more time to cool because of the higher ambient temperature. Test two with the cardboard should have cooled in less time because of the lower ambient temperature. These effects should have minimized the time difference between the tests. Yet these two tests show nearly the greatest |
|
| Back to top |
|
 |
mcstargazer
Joined: 07 Nov 2006
Posts: 4
|
 Posted: Tue Nov 07, 2006 11:41 pm Post subject: Addendum: this text was lost from the previous post Posted: Tue Nov 07, 2006 11:41 pm Post subject: Addendum: this text was lost from the previous post |
 |
|
...nearly the greatest difference in times with the cardboard providing a 26% gain in heat retention time over the wood.
I think these preliminary tests are encouraging. More work should be done. Any takers? Please see the list of possible enhancements below for ideas, or come up with your own!
***** Further Enhancements? *****
-Add layers of aluminum foil between each layer of cardboard to incorporate a radiative heat shield into the block.
-Hollow out the block by cutting the centers out of each layer, except the top and bottom layers which should be left to keep the block structurally rigid. This may reduce conductive heat losses through the cardboard.
-Perhaps only hollow out every other layer of cardboard, yet leave each sheet of aluminum foil intact? This may provide better structural stability with more small dead-air spaces offering less chance of convective heat transfer through the block.
-Come up with an appropriate way to seal the cardboard against moisture so that it can be placed in the bag with the pot.
-Can someone offer better control of the ambient temperature and run a statistically significant number of tests?
-Can someone offer actual tests under cooking conditions? |
|
| Back to top |
|
 |
webmaster
Site Admin
Joined: 25 Oct 2006
Posts: 66
Location: Seattle, Washington
|
 Posted: Tue Nov 07, 2006 11:53 pm Post subject: Did you place the block inside the cooking bag or outside? Posted: Tue Nov 07, 2006 11:53 pm Post subject: Did you place the block inside the cooking bag or outside? |
 |
|
| Hi. Thanks for being our first post in our new forum system. Was the base inside or outside of the bag? I think this would make a difference in the actual gain here. |
|
| Back to top |
|
 |
mcstargazer
Joined: 07 Nov 2006
Posts: 4
|
 Posted: Wed Nov 08, 2006 12:19 am Post subject: Posted: Wed Nov 08, 2006 12:19 am Post subject: |
 |
|
| You are correct. Theoretically, the bag will make a difference in that it would provide dead air space around the sides of the insulating block and slightly improve the efficiency. However this improvement would be made to both wood and cardboard blocks alike if placed inside the bag. With all other things equal, I only hoped to show with the experiments that either the cardboard or the wood was a superior thermal insulator. After establishing which is better, it would help to place the winner inside the bag for even greater efficiency. Thanks for helping me clarify the discussion. |
|
| Back to top |
|
 |
David Whitfield
Joined: 08 Nov 2006
Posts: 9
Location: Bolivia
|
 Posted: Fri Nov 10, 2006 2:03 pm Post subject: variations to consider Posted: Fri Nov 10, 2006 2:03 pm Post subject: variations to consider |
 |
|
Hi Star gazer,
Why not try keeping the insulator on the outside of the bag, but raise the pot on some small blocks to give a bottom airspace inside the bag.
On reason I suggest keeping the insulator outside is the moisture factor. The water droplets will reduce insulation ability of either unless they are sealed against moisture.
I expect that the cardboard gives better results (and they were significant results!) because the cells and air spaces between them were less dense that the wood.
Great work
keep looking up
David
_________________
Skpe - solar1bol |
|
| Back to top |
|
 |
alexkee
Joined: 12 Nov 2006
Posts: 3
Location: Malaysia
|
 Posted: Mon Nov 13, 2006 2:46 am Post subject: Egg Carton: Simple & Effective Solution to Conduction Lo Posted: Mon Nov 13, 2006 2:46 am Post subject: Egg Carton: Simple & Effective Solution to Conduction Lo |
 |
|
Conduction, Convection and Radiation, three ways of thermo-dynamics transfers. The block of wood vis-à-vis cardboard block may surprise most people that actually air and vacuum are better thermal insulators for thermal conduction and even convection, but not radiation loss. Thermal Radiation Loss is conserved through reflectance like the silver lining on the outer-side of any co-axial Thermos or Dewar Vacuum Flask.
For one, according to C.D. Hodgman (ed), Handbook of Chemistry and Physics,, The Chemical Rubber Co., Cleveland, 1942, the thermal conductivity (measured in kcal-cm/(s))cm2)(°C))of Air is 0.0000568 whereas cardboard and wood (fir) is 0.0005 and 0.0003 respectively.
Another critical consideration is moisture, water has a thermal conductivity factor of 0.00138 and 0.00143 at 4°C and 20°C respectively, so it is best to keep the insulator as dry as possible.
Maybe, the simplest, cheapest and most cost effective material to use for this purpose is paper type egg carton which are easily available, strong enough to take the weight of most filled pots and yet have plenty of air spaces.
The wind shear cooling factor should not be underestimated and therefore, there is also a tradeoff between conduction loss and convection loss when winds are strong and constant.
_________________
The Sun, Humanity's Ultimate Equalizer |
|
| Back to top |
|
 |
David Whitfield
Joined: 08 Nov 2006
Posts: 9
Location: Bolivia
|
 Posted: Mon Nov 13, 2006 7:21 pm Post subject: Posted: Mon Nov 13, 2006 7:21 pm Post subject: |
 |
|
wow Alex,
You are full of important technical data. Great contribution!!
Good to hear from you
David
_________________
Skpe - solar1bol |
|
| Back to top |
|
 |
mcstargazer
Joined: 07 Nov 2006
Posts: 4
|
 Posted: Tue Nov 14, 2006 5:33 pm Post subject: Posted: Tue Nov 14, 2006 5:33 pm Post subject: |
 |
|
A few comments on moisture and "the numbers" to keep the discussion rolling...
MOISTURE
An important idea that I am learning from the previous posts by David and Alex, and the US Forest Service document referenced below is that moisture can rob a material of its thermal insulating properties. Thank you!
I like the idea of keeping the block outside of the bag in order to keep the moisture away from it. I still think it would be a good idea to learn how to seal it against moisture though. Perhaps baking the block in a solar oven and then quickly sealing it with an appropriate material could increase the insulating value by removing much of the moisture content from the interior of the block?
THE NUMBERS
While the numbers can inform experiments for those with a scientific background to use them, a healthy caution must be maintained.
A single number for the coefficient of thermal conductivity for wood isnt useful as the real world is complex and non-linear and can rarely be explained with simple numbers. As a case in point, look at this US Forest Service publication http://www.fpl.fs.fed.us/documnts/fplgtr/fplgtr113/ch03.pdf and find the section on the thermal conductivity of wood. There you will find that it varies according to density, moisture content, extractive content, grain direction, structural irregularities, and temperature. Thus, a single number cannot be used to extrapolate the thermal conductivity of all woods under all conditions. A quick look at coefficients in data tables could mislead experimenters.
A further caution with regards to numbers concerns the context of the given number. According to the post above, a quick look at the Handbook of Chemistry and Physics implies that cardboard is a poorer insulator than wood (or at least fir wood anyway), and would dissuade experimenters from trying out cardboard products. However, the Handbooks numbers run contrary to my preliminary experiments. What is happening? I suspect that the value given for cardboard is for a straight sheet and not for corrugated cardboard which has dead air spaces in the channels. I tried looking in my 1970 edition of the Handbook to confirm my suspicion, but there were no entries for cardboard.
Ultimately, we are looking for real world materials working in real world scenarios that can be duplicated in developing regions. With my background in physics Ive learned that theory can often be used to inform experiments, but things dont always turn out like you expect. And thats why we experiment. We need people to jump in here and try things out. Try out the egg carton suggested by Alex, try out the modifications I listed in the second posting above, try out whatever is in your junk room and see what works. Perhaps, if I can find time over the holiday break between semesters, Ill do this very thing and continue experimenting. |
|
| Back to top |
|
 |
Ramon
Joined: 31 Oct 2006
Posts: 3
Location: Sacramento, California
|
 Posted: Wed Nov 22, 2006 10:42 pm Post subject: Improved block of wood Posted: Wed Nov 22, 2006 10:42 pm Post subject: Improved block of wood |
 |
|
You are having an interesting discussion on improving the block of wood, but I am not all that clear why one uses a block of wood to support a pot in the CooKit. SCI originally suggested elevating the pot above the floor of the CooKit (to keep heat from conducting from the bottom of the pot, through the bottom of the CooKit into the nearly infinite heat sink of the Earth) by using 3 or 4 flat stones or a couple of slender sticks. This simple measure does prevent most of the conduction you are worried about. Plus, it allows heated air to circulate below the pot. A few sticks or flat stones are a little awkward to use, I agree. Steven Jones of Brigham Young has investigated other ways of raising the pot, and while there may be some stability issues, there are also gains to be had from his methods--namely, getting reflected sunlight to shine on the pot bottom. You can find an article of his that tells more at this URL--http://solarcooking.org/saveheat.htm
_________________
Ramon Coyle
"keeping signature blocks simple since 2006" |
|
| Back to top |
|
 |
webmaster
Site Admin
Joined: 25 Oct 2006
Posts: 66
Location: Seattle, Washington
|
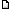 Posted: Thu Nov 23, 2006 1:41 am Post subject: Photo of Steven Jone's support Posted: Thu Nov 23, 2006 1:41 am Post subject: Photo of Steven Jone's support |
 |
|
 |
|
| Back to top |
|
 |
MarkS
Joined: 19 Nov 2006
Posts: 1
|
 Posted: Fri Nov 24, 2006 4:07 am Post subject: Re: Improved block of wood Posted: Fri Nov 24, 2006 4:07 am Post subject: Re: Improved block of wood |
 |
|
| Ramon wrote: | | SCI originally suggested elevating the pot above the floor of the CooKit (to keep heat from conducting from the bottom of the pot, through the bottom of the CooKit into the nearly infinite heat sink of the Earth) by using 3 or 4 flat stones or a couple of slender sticks. |
Yes, its possible that if the cardboard works better than the wood its because the cardboard is not as good an insulator as the wood. The only way for heat to reach the bottom of the jar/pot in most cookers is by air circulation under the container. So the question is not whether wood is better than cardboard but whether either work as well as simply elevating the container. |
|
| Back to top |
|
 |
Bill Bradley
Joined: 26 Nov 2006
Posts: 21
Location: Springfield MA USA
|
 Posted: Tue Nov 28, 2006 12:43 am Post subject: Posted: Tue Nov 28, 2006 12:43 am Post subject: |
 |
|
There is another reason for raising the pot that has not been mentioned. Some sun reflects from the bottom part of the cooker and hits the bottom of the pot provided that the sun is not directly overhead.. A solid material such as wood or cardboard laminate defeats this source of heat gain. A simple wire trivet may be a more effective way of raising the pot than a wood or cardboard block.
Bill Bradley
_________________
Bill Bradley, EarthboundTech |
|
| Back to top |
|
 |
DerekPearcy
Joined: 15 Sep 2007
Posts: 9
Location: Shelbyville, IL
|
 Posted: Sat Sep 29, 2007 1:10 pm Post subject: Posted: Sat Sep 29, 2007 1:10 pm Post subject: |
 |
|
I like the idea of the wire mesh to raise the pot really. Thats easy and light. But I guess if you dont have a roll of wire mesh, you'd have to buy a whole roll just for that wouldnt you?
Another thing I have used is from the craft store and less than $1. Its the green styrofoam blocks that are used for flower arrangements. They insulate pretty well and are very light and cheap. I bought a circular one and it works pretty well.
http://www.FreeEnergySolutions.net
_________________
http://www.FreeEnergySolutions.net |
|
| Back to top |
|
 |
|



